

During the brief passage of solar radiation through the Earth's atmosphere, certain frequencies are selectively absorbed. Since each different atmospheric gas has its own unique absorption pattern, determined from laboratory measurements on pure gas samples, it is possible to determine which gases are present in the atmosphere and quantify their abundances. To date, over 30 different gases have been measured using this technique including H2O, CO2, O3, N2O, CO, CH 4, N2, O2, NO, NO2, HNO3, HNO4, N2O5, ClNO3 , HOCl, HCl, HF, SF6, COF2, CF4, CH 3Cl, CHFCl 2, CFCl3, CF2Cl 2, CCl4 , OCS, HCN, CH3CN, HCOOH, H2CO, C2H2, C2H6 and many isotopic variants.
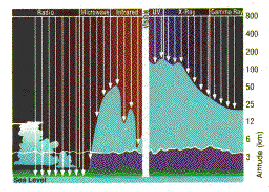
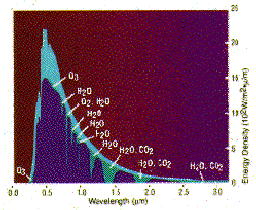
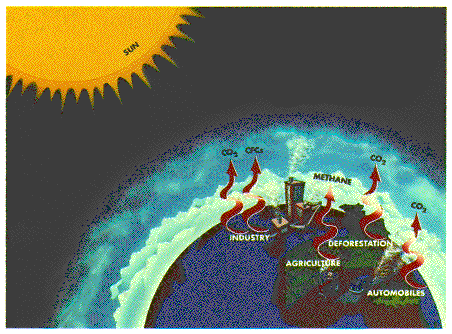
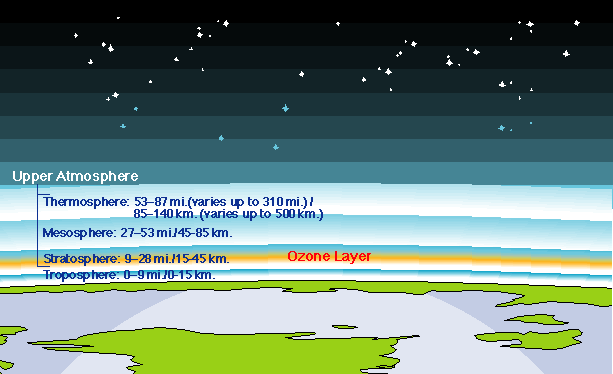
Ozone is created and destroyed by complex reactions involving ultraviolet radiation from the sun and gases in the middle atmosphere. While some of those gases occur naturally, concentrations of destructive chemicals are increasing due to human activity. To fully understand the many factors that drive atmospheric chemical reactions and to predict changes, scientists must have a comprehensive knowledge of the gases which make up the atmosphere.
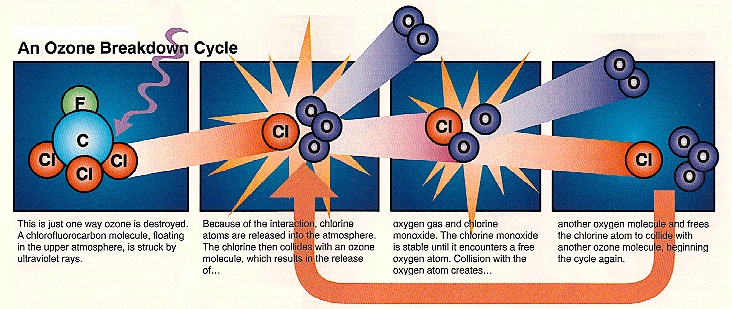
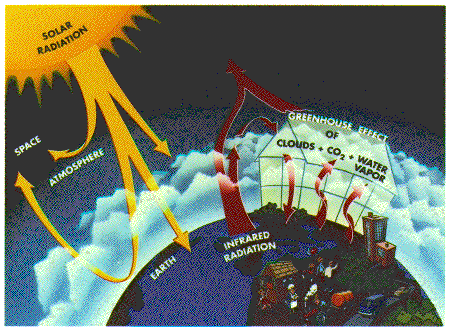
 Go back to the
previous page
Go back to the
previous page